Insights

Health economic modelling: a guide for pharmaceutical and medical device companies
Health economic modelling is a process for synthesising and comparing the costs and benefits of new (proposed) and existing medicines or devices in order to make decisions about which interventions may lead to the best patient outcomes. It is a key component of the health economic evaluation process. Around the world, health economic modelling is a “must” for manufacturers who need to articulate the value of their new health intervention and seek market access.
An introduction to health economic evaluation
The purpose of health economic evaluation is to support decision-makers in allocating limited healthcare resources to achieve the best possible outcomes for patients. Deciding to invest in one health intervention means not investing in another. Decision-makers will be willing to invest in a health intervention only if its value exceeds that of the alternatives. A cost-effectiveness model draws information from various sources to help analyse which intervention represents the best value for the decision-maker.
There are several types of economic evaluation, all of which synthesise information about patient health and associated costs in order to inform decision making. The types of evaluation differ in how they account for the costs and benefits of an intervention (see table below).
Types of health economic evaluation

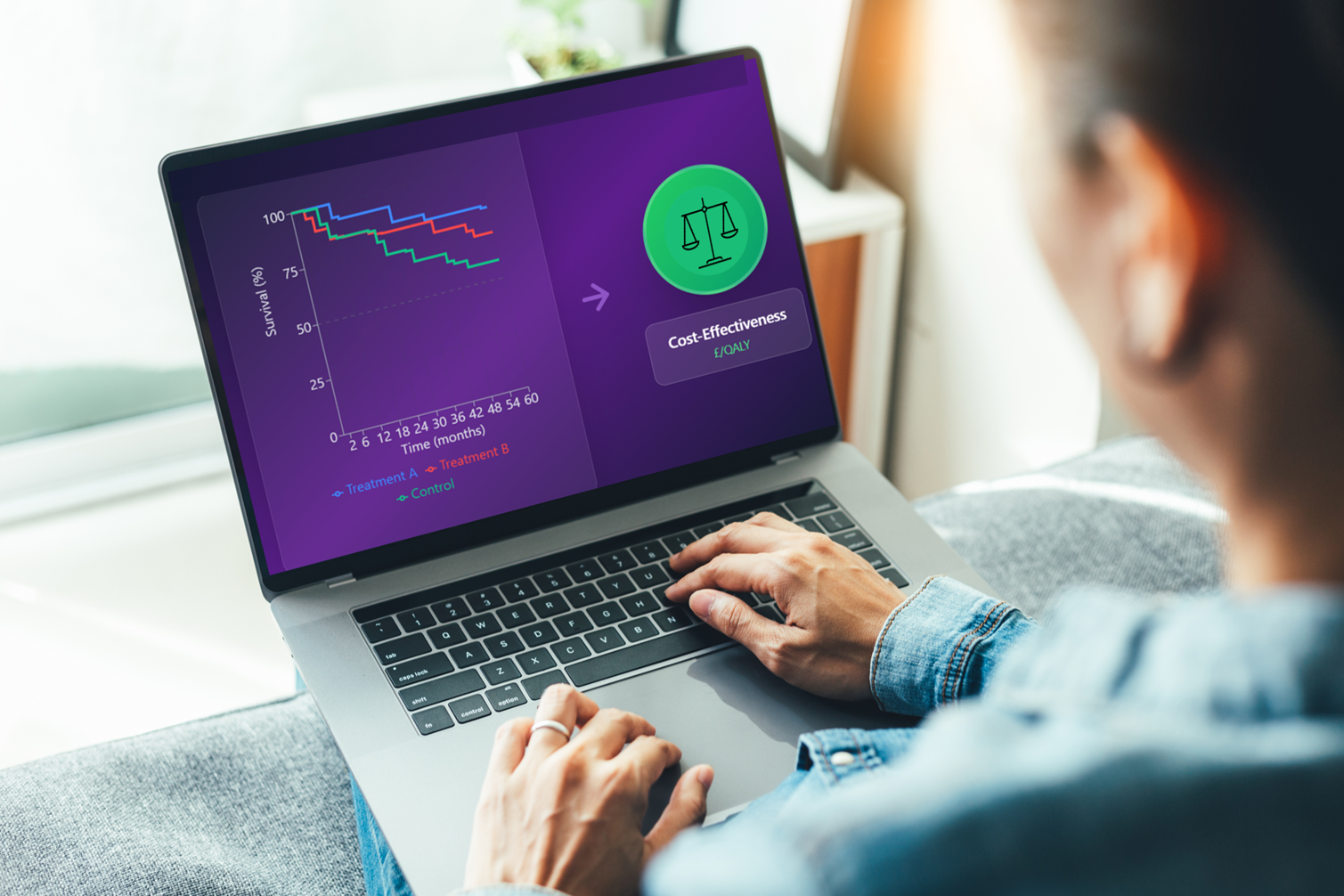
Health economic modelling for cost-effectiveness analysis
Deciding whether to invest in a healthcare intervention in an informed way means processing large amounts of data – e.g. about costs, efficacy, safety, and quality of life – for a new intervention and existing alternatives. Health economic modelling aims to synthesise all this information into a single set of calculations. At Symmetron, cost-effectiveness analyses are the type of economic evaluations that we conduct most frequently, as these are required to secure reimbursement from NICE and other healthcare payers. For the cost-effectiveness analysis, we typically develop health economic models in Microsoft Excel, though we sometimes use other software platforms such as R.
There are many types of economic models for cost-effectiveness analysis, some of which are summarised in the table below. Which type of model is the most appropriate depends on the complexity in capturing the impact of the intervention, the availability of data, and the requirements of the decision-maker.
Types of economic models for cost-effectiveness analysis

Achieving market access
Bringing a new medicine or device to the market typically involves several stages of economic modelling. Modelling usually begins towards the end of product development because data must first be generated in order to construct a reliable model. Nevertheless, modelling can also be conducted earlier during development to provide initial estimates about cost-effectiveness. This early modelling can help pharmaceutical companies identify key drivers of the value of their medicine or device. Understanding drivers of value can inform research and market access by ensuring that the key data are captured and the intervention is targeted at the appropriate group of patients.
When phase III trial data and/or real-world evidence become available, they can be incorporated within an existing model or used to inform a new, more comprehensive model. The economic model can then be used to apply for reimbursement, pursue academic publication or inform decision making. The key is ensuring that the model is fit for its purpose.
Health economic modelling and Symmetron
We at Symmetron will work with you to create health economic models that meet your specific needs and follow best practices, based on our own experience and our close attention to industry trends.
- We recommend that you engage with health economics and outcomes research (HEOR) experts early in order to understand the available evidence, the care pathway and similar treatments already available.
- We identify key drivers of the value of a drug or device in order to ensure that the relevant evidence is collected and highlighted.
- We identify any additional evidence that may be required to develop a robust economic model, and we generate this evidence where necessary.
- We collaborate effectively with diverse stakeholders, including client teams, clinical experts, academic researchers, and other professionals.
- We co-produce economic models with end users in mind, ensuring that non-experts can easily use them.
- We understand how to design economic models that meet the requirements of various audiences, including health technology assessment (HTA) agencies, academic journals, and local decision makers.
The health economic models that we develop are:
- Tailored to your needs
- Informed by the best available evidence
- Reflective of global best practices
- Expertly validated
- Designed to meet user requirements
- Impactful for decision making
Across all our activities, we at Symmetron are driven by the goal of increasing the number of effective treatments available to patients. We are proud to help clients bring the latest scientific advances to patients in stride with the ever-changing healthcare landscape. Partner with our team by contacting us here.
Similar Insights
Stay in touch
Subscribe to Symmetron and stay up to date with recent news and announcements.






.jpg)



















